Dynamic Flow Vitamin C

Recent columns have focused on our expanded knowledge of vitamin C's health benefits. The over-riding message has been, "Vitamin C is helpful, in higher doses it is highly effective." This month, we are consulting with Dr. Steve Hickey on maintaining desired blood and immune system levels of vitamin C. This is primarily for those individuals wishing to increase their protection against viruses and other health threats or wishing to maintain increased blood levels of vitamin C between courses of Intravenous (IVC) Vitamin C medical treatments. However, it applies to general nutrition as well. It's not how much you put in your mouth, but how much gets into your blood and to your cells. What should your blood level be for good health? For optimal health? For adjunct therapy for disease states?
We will also discuss the misleading "information" being widely quoted and disseminated that the blood and/or body reaches a vitamin C saturation with doses under a gram (1,000 mg). Not so! The problem is that this misconception encourages the use of low doses and hinders the research on high-dose vitamin C as an adjunct therapy that could save many lives. Just as important, it also serves to discourage research on the subject. This misconception must be corrected. The aim of scientific research is truth.
We will also discuss the advantages of taking vitamin C supplements in split doses as opposed to all at once. Ever wonder why different organs in the body hold different amounts of vitamin C and why do these amounts change when the body is under attack or stress?
Does research suggest that there is a minimum blood level of vitamin C that must be exceeded to begin the cancer-cell killing process? Or does research suggest that any level of vitamin C can kill some cancer cells in proportion? If the former, what is the required level, and can it be reached via oral supplementation as well as IV infusion?
Research has also documented that cancer cells consume significant amounts of vitamin C, thereby robbing the body and increasing the risk of pre-scurvy in cancer patients.
Readers, please keep in mind that this interview is a discussion between two scientists about the ongoing research on vitamin C (ascorbic acid) and should NOT be construed as offering medical advice to anyone. Information in this interview is intended for educational and scientific purposes only. It is not intended as medical or nutritional advice for the treatment or prevention of disease. In the United States, the Food and Drug Administration regulations state that any claim that a nutrient treats a disease, immediately, by definition, changes such nutrient into a drug and subject to all drug regulations. We will be discussing ongoing scientific research, not discussing medical advice. For medical advice, please consult your personal health care practitioner. Nonetheless, you may find our discussion of this ongoing research interesting.
An important aspect of vitamin C nutriture is that it is highly water-soluble and is quickly removed from the blood by the kidneys. In biochemistry, the time required for a quantity of a compound to be reduced to half its initial value is known as the "biological half-life" of a compound. Unlike the decay of radioactive compounds — which is purely exponential — biological half-lives are more complex and describe the time that it takes for the concentration of a substance in blood plasma to reach one-half of its steady-state value (the "plasma half-life"). The relationship between the biological and plasma half-lives of a substance can be complex, due to factors including accumulation in tissues, active metabolites, and receptor interactions.
Since vitamin C is highly water-soluble it is readily excreted in urine and should be consumed periodically throughout the day to maintain desired blood levels at all times. Dr. Robert Cathcart has found that the amount of vitamin C absorbed is not constant but depends on need (1). Dr. Steve Hickey and his colleagues have determined that the plasma half-life for high doses of vitamin C is about 30 minutes in well-nourished, normal healthy individuals (2). However, while it is being excreted, it can be replaced by absorption from the gut or by redistribution from other body compartments. When there is vitamin C deficiency, the kidney will attempt to re-absorb more of the vitamin C and put it back into the plasma. This is a "dynamic flow" rather than a steady fixed value. As my youngest son Michael points out, Dynamic flow is a critical concept — we seem to grasp that we shouldn't try to inhale a day's supply of oxygen in one sitting, or drink a day's supply of water/fluid in one sitting; why do we think we should consume a day's supply of vitamin C in one sitting? When we work harder, we breathe deeper and faster, when we are stressed we should take more vitamin C.
In 2005, based on their biochemical and clinical findings, Drs. Hickey, Roberts and Cathcart formulated the Dynamic Flow model for absorption and action of vitamin C in the body (3). The dynamic flow model refutes the current low-dose recommendations for vitamin C intake and supports megadoses of vitamin C for the adjunct use for disease conditions. We will discuss the meanings of all of this later.
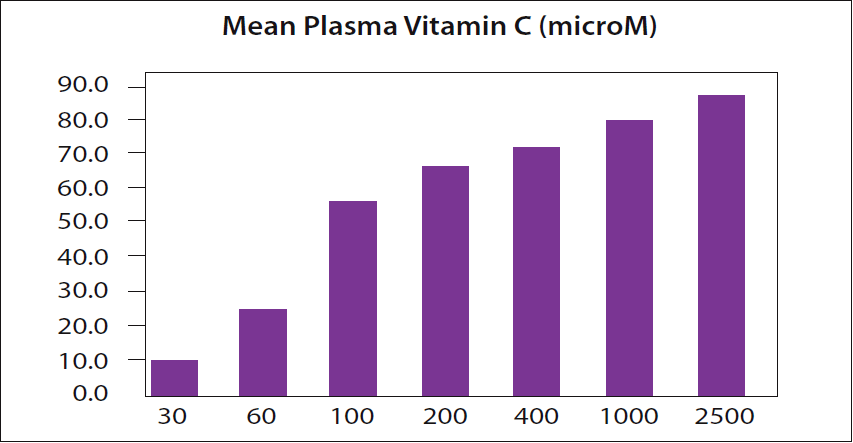
because, as their own data show, the plasma vitamin C level for a 2,500 mg dose is even higher. The level of vitamin C in the plasma is expressed in micromoles (microM or µM). Data from page 82 of reference 6.
In a recent column, I discussed Dr. Hickey's point that there is a widely quoted dogma from a 1996 NIH study which claimed that the blood can become "saturated" with vitamin C at a single dose of 200 milligrams (4). Dr. Hickey had pointed out that this 1996 NIH study's own data even showed this is not so (5). The figure that I included in the March 2018 column is shown again as Figure 1 here. It is based on page 82 of reference 6.
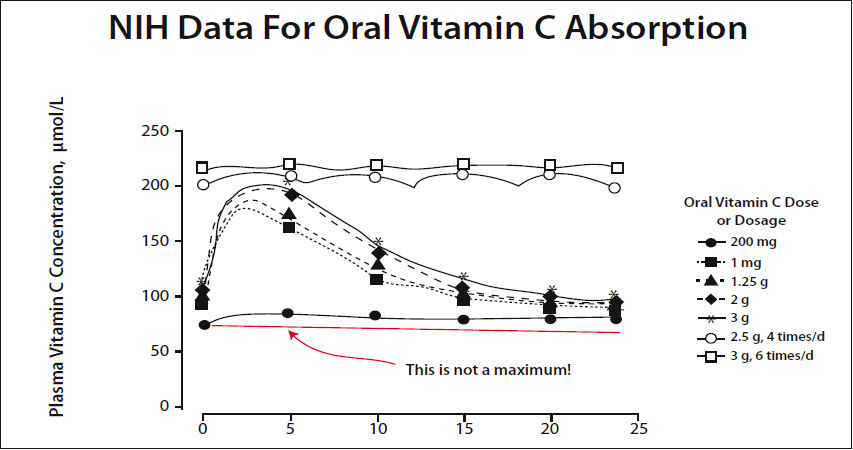
In a newer graph prepared by Dr. Hickey using the same NIH data, the point is made very clear. A 200 mg dose is not the maximum amount that can be absorbed. (Please see Figure 2.)
Dr. Hickey will discuss this point in this column.
Dr. Hickey, after studying mathematics and science at the Open University and pharmacology at Manchester Metropolitan University, earned his Ph.D. in medical biophysics from the University of Manchester. Dr. Hickey has coauthored several books including "Vitamin C: The Real Story" (with Dr. Andrew Saul, 2008), "Ascorbate: The Science of Vitamin C" (with Dr. Hilary Roberts, 2004), "Cancer, Nutrition and Survival" (with Dr. Hilary Roberts, 2005), "Ridiculous Dietary Allowance" (with Dr. Hilary Roberts, 2005), "The Vitamin Cure for Migraines" (with Dr. Andrew Saul, 2010), " The Cancer Breakthrough" (with Dr. Hilary Roberts, 2007) and "The Vitamin Cure for Heart Disease" (with Dr. Hilary Roberts, 2011).
Passwater: Welcome back! Dr. Hickey, previously, we have discussed "The Science of Vitamin C and Cancer" and "The Inappropriateness of So-Called 'Evidenced Based Medicine' — Especially for Studying Nutrients: A Tarnished Concept" in this column (8,9).
In December 2007, we discussed "The Science of Vitamin C and Cancer" (8). That was 11 years ago. In that column it seems as if we were discussing the same main points that we will review herein. Hasn't the scientific community learned that the data often quoted on vitamin C absorption and the amount of vitamin C that the blood can hold is wrong? Is that because the incorrect data was published by the National Institutes of Health (NIH) or what? Could it be that they don't want to change their dogma?
Hickey: Yes. The so-called authorities have a thing about vitamin C. It became evident when Dr. Linus Pauling tried to make the case for gram- level intakes several decades ago now. Despite his eminence, Pauling was subject to public abuse and castigation despite the data he presented. This strange reaction about the vitamin continues to this day.
The NIH continues to promote the low-dose dogma despite a great deal of public embarrassment over their errors. They have simply waited for people to forget and changed a few descriptive terms. The phrase "tightly controlled" was substituted for "saturated," for example. One improvement is that they were forced to switch from a maximum blood level of only 60-70 µM/L to 200-250 µM/L. What they are still hiding is that their own data show these higher levels require a dynamic flow intake of about 20 grams a day rather than 200 mg.
Passwater: Surely other scientists working in nutrition would have corrected the error?
Hickey: It is indeed a strange situation. For over a decade, the NIH papers were quoted widely, described as uniquely rigorous, and used for specifying the RDA. There is what we might call a core NIH supporters group. Even now, years after the errors were made clear, peer reviewed papers report on the NIH work as if there were no problems. This continuation is despite the public embarrassment for the NIH when the mistakes became public knowledge.
Passwater: What are the implications for the typical person of a recommended intake based on the NIH recommendations?
Hickey: The NIH have data for young healthy middle-class adults. So, if you are in this category and are a non-smoker, not sick, or stressed, then 200 mg a day will prevent you from getting acute scurvy. However, their data do not cover someone who is under stress, ill, or aging. Importantly, they have no data on the long-term effects of a higher intake of vitamin C. Many of the major chronic diseases of the West, such as heart disease, arthritis, and cancer, could be a result of inadequate intake. We just don't know.
Dr. Robert Cathcart explained a phenomenon of massively increased absorption when a person is sick or stressed. The maximum tolerated intake can go from, say, 2 grams a day when a person is in good health to 100 grams when they are sick. This huge increase is consistent with a similar rise in a person's needs. He observed that the symptoms of many illnesses are improved at these high levels. The NIH and other mainstream researchers simply ignore Dr. Cathcart's work.
Passwater: How do Dr. Cathcart's observations and those of other nutritional doctors compare with the mainstream claims that clinical trials have shown megadose vitamin C to be ineffective as a cure for the common cold?
Hickey: The mainstream clinical trials on vitamin C and the common cold or other infections have generally used doses around one gram a day and often less. Physicians such as Robert Cathcart or Abram Hoffer did not claim that a gram (or 1,000 mg) would cure a cold. They observed that intakes in the region of 10 grams (10,000 mg) would prevent most colds but that treating a cold could require 50-100 grams (50,000-100,000 mg). In other words, the trials confuse by calling a small nutritional intake a "megadose" and avoiding the issue. People are being hoodwinked.
One notable thing is that the NIH are promoting an idea for the use of IVC in cancer treatment. They state that only IVC could be helpful in cancer as supplements could not work. In doing so they compound their initial errors by adding more mistakes.
For example, they inaccurately claimed that the successful clinical trials of vitamin C in cancer by Ewan Cameron, Linus Pauling and others were done with IV. The failure of the Mayo Clinic to repeat the successful studies was supposedly because they used oral vitamin C.
The initial trials using oral vitamin C in cancer gave astounding results. Patients lived far longer. Dr. Abram Hoffer gained similar results to Cameron and Pauling as did a group of doctors in Japan (10). Importantly, these successful trials used oral vitamin C, or oral vitamin C occasionally supplemented by IV. Dr. Cameron was quite specific and stated that IVC provided no additional benefit unless the patient was unable to take the vitamin by mouth because of vomiting.
The NIH emphasize the massive concentrations that can be briefly achieved in the blood using IV. High levels are obviously possible as the vitamin is being poured directly into a vein. In taking this macho IV approach they have forgotten one of the first rules of pharmacology. The effect of a substance depends on the dose, duration and frequency of administration. In other words, how often you take a drug and for how long can be as important as the size of the dose. I know saying it like this is obvious but apparently it needs restating.
Passwater: Each of the parameters is important. It's like a three-axis graph, with the effective area in the middle and the ineffective areas along the edges.
OK, this is the crux of this discussion, so let's be as clear as possible for our readers, even if we must be redundant. Is it your contention then that the scientific literature supports that oral vitamin C can be safely consumed at a level that is at least as effective as IVC? As a follow-up, is it your contention that the science suggests that oral vitamin C can be even more effective that the current IVC protocols?
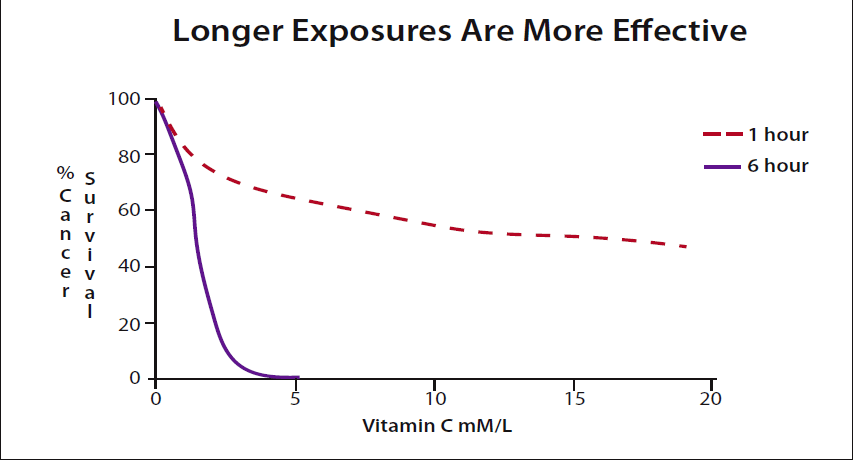
Hickey: Yes. Essentially IVC will increase blood levels to a very high value for a short period, a few hours. However, oral intakes can sustain high blood levels. In other words, oral doses can deliver a far greater total amount of vitamin C to a tumor than can be achieved practically using IV. I can find no data supporting the contention that IVC is a more effective cancer treatment than oral vitamin C. Given some minimum effective concentration, the longer the exposure, the more cancer cells are killed. (Please see figure 3.)
Passwater: Just to be clear as possible for our readers, is it your contention that the scientific literature supports the premise that it is simply a matter of killing more cancer cells with vitamin C as the cancer can replicate (as opposed to the effectiveness being that a threshold blood level is being reached)?
Hickey: The important thing is to maintain the pressure on the cancer so that it can't grow and adapt to the treatment.
Passwater: Can multiple doses of high amounts of vitamin C raise the plasma level of vitamin C sufficiently to kill cancer cells?
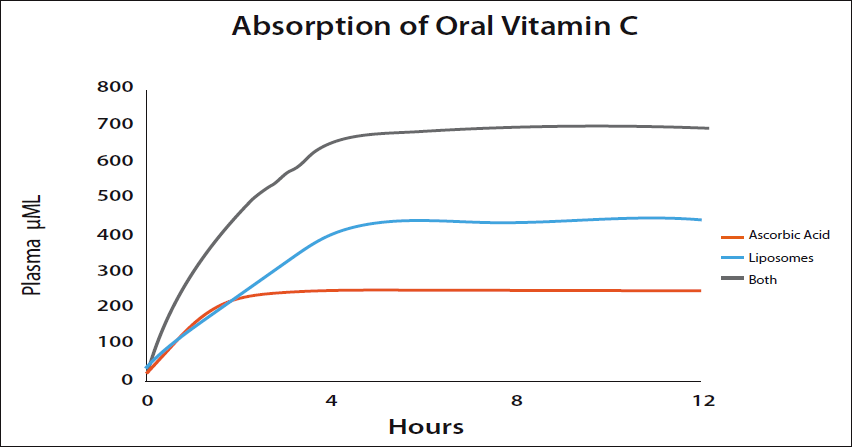
Hickey: With standard ascorbic acid taken orally the maximum plasma level seems to be in the region of 250 µM/L. Biochemists usually express the concentration of a solute (in this case vitamin C) in the blood in terms of molarity such as micromoles (µM) per liter. The advantage is that it's easy and convenient to use because the solute may be measured in grams, converted into moles, and mixed with a volume. The molarity (M) is defined as the number of moles of solute per liter of solution. A healthy adult might attain this level for a short period with a single dose of about five grams. Liposomal vitamin C is a little more efficient. Liposomal vitamin C surrounds the vitamin C molecules in phospholipids and carry the vitamin C into the blood by bypassing the normal absorption route. Plasma levels of 400 µM/L or more can easily be achieved using liposomal vitamin C. The limiting factor is the phospholipid which has its own bowel tolerance level.
Standard vitamin C (ascorbic acid) and liposomal vitamin C are absorbed by different mechanisms and are approximately independent. Taking both together leads to the blood levels being added. This adds the 250 from ascorbic acid to the 400 or so from the liposomes giving 600-700 µM/L. These levels are high enough to kill cancer cells. (Please see Figure 4.)
Passwater: This appears to contradict NIH claims that oral vitamin C will not help cancer patients and IV is necessary.
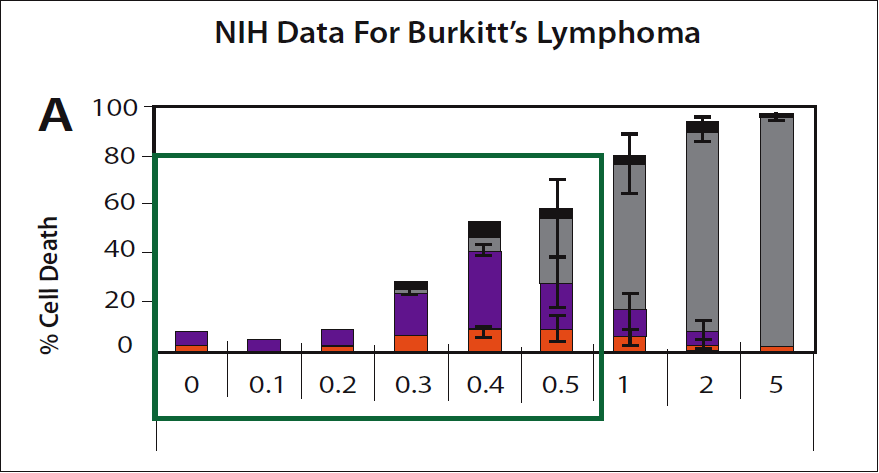
Hickey: For fun we can use the NIH data to show once again that they are wrong. They provide a chart to indicate the killing of Burkitt's lymphoma cells by increasing concentrations of vitamin C. (Please see figure 5.) It shows how a very short exposure to dynamic flow levels of vitamin C can kill cancer cells. The chart shows that 0.3 mM/L (300 µM/L in the units we are considering) kills these cancer cells. Importantly, adding some other supplements increases the selective cancer killing of vitamin C, for example alpha-lipoic acid enhances the effect by a factor of 5-10 times.
Passwater: You have been careful to say a "very short exposure." Is something else being hidden?
Hickey: Yes. Typically, the experiments used to test selective killing of cancer cells only expose the cancer to vitamin C for a single hour. The NIH are explicit and indicate that they are considering IV which is administered over a similar short interval.
As you might expect, more cancer cells are killed the longer they are in contact with the vitamin. The effect is quite dramatic. (Please see figure 3.) Extending the exposure for only a few more hours shows that oral vitamin C may be far more effective than IV. This is what Cameron and the others found and why the recent IV studies have failed to show the same benefits.
I cover this in a new book "Vitamin C and Cancer" (published by CreateSpace) that will be released later this year.
Passwater: Can cancer cell killing levels be sustained?
Hickey: Yes. With repeated doses the levels can be sustained indefinitely. An IV infusion lasts a few hours, but oral dynamic flow can continue for years. Repeating doses a few hours apart maintains the blood levels.
Passwater: One of your books with Dr. Hilary Roberts, "Ascorbate: The Science of Vitamin C," is still the most informative book on vitamin C that I have read (6). There are several excellent books of vitamin C, but if I had to recommend just one book, it would have to be yours. Thanks for writing it. How did it come about?
Hickey: Following Dr. Linus Pauling's death, Dr. Hilary Roberts and I were concerned that misinformation about vitamin C had increased and had not been challenged properly. At that time, it was generally accepted that NIH had shown that the body did not absorb high intakes. It was clear that there was a contradiction between this and claims for high-dose vitamin C. If only low doses are absorbed, then the claims must be false.
The NIH published their research shortly after Pauling's death and met with little critical response. Although few nutritionists believed the NIH results, there was apparently no reason to doubt their validity. If the NIH were correct, however, Pauling and the orthomolecular physicians had been misled and high-dose vitamin C would be useless.
Around the 10th anniversary of Linus Pauling's death, we decided to review the current evidence on the benefits of vitamin C. Our aim was to bring more rationality into a subject characterized by prejudice and lack of objectivity. We wanted to explain the discrepancy between the orthomolecular claims for vitamin C and the experimental work, which apparently indicated it was not absorbed at high doses.
Passwater: Well, that's certainly important. The Dynamic Flow model for vitamin C developed by yourself and Drs. Roberts and Cathcart brings together evidence from both viewpoints and resolves the apparent contradictions (3).
Hickey: Linus Pauling did a lot to popularize the claims for high doses of vitamin C in preventing and treating infections, heart disease and cancer. However, current medical thinking was inconsistent with clinical observations on high-dose vitamin C. Dr. Archie Kalokerinos, in his 1974 book "Every Second Child," describes using high-dose vitamin C to bring sick children back from the brink of death, within minutes. Dr. Ian Brighthope states that vitamin C could reverse AIDS. Drs. Pauling, Cameron and Hoffer presented data showing that vitamin C prolongs the lives of cancer patients. Multiple independent physicians replicated these effects, which seemed to show that high-dose vitamin C could have astounding results.
If these powerful claims for vitamin C were correct, the NIH claims that the body was saturated at a low dose must be wrong, or vice versa, since the two ideas were incompatible. In science, contradictions and paradoxes indicate areas where we are ignorant of the underlying mechanisms and show where progress can be made. To us, the contradiction was an indication that there was fun to be had, getting to understand the workings of vitamin C in the human body.
Passwater: As I mentioned earlier, more than 11 years ago, you had found flaws in the "official" RDA for vitamin C and other nutrients. You asserted then that the RDA justification for low intakes of vitamin C was both invalid and indefensible. I promised that we would get back to this later. Let's look at the details now.
Hickey: It had become apparent that the NIH's work on vitamin C pharmacokinetics was confused. Indeed, their own data indicated that a healthy person could consume at least 18,000–20,000 mg/day and get a corresponding increase in blood plasma levels (5). We tried explaining this to the NIH researchers, but they did not seem to understand.
The NIH publications misinterpret their data. Their results showed clearly that the blood plasma was not "saturated," at low doses as they suggested. It seemed to me that they were so keen on showing Linus Pauling to be wrong that they were unable to see what their data implied.
Vitamin C is unusual in that, at low intakes, it has a biological half-life of 8–40 days. The kidneys actively prevent it from being excreted. When vitamin C is in short supply, this is an essential mechanism for animals such as humans that do not synthesize the vitamin internally. At high doses, however, the biological half-life is only about 30 minutes. Given this fast excretion time, the NIH had waited too long to measure the blood levels. By the time they took their measurements, the vitamin C dose had been excreted, so the plasma level did not increase greatly with the dose. Instead of realizing that a once or twice daily dose interval was too long, they thought the body had become saturated.
We gave the NIH a year to consider the need for a correction or to support their saturation statements. They refused to collaborate on a paper to amend the claim. When they did not respond, we made the error known to the public.
Passwater: Even the National Academy of Sciences 2000 RDA publication stated that quantities of vitamin C above a gram (1,000 mg) are absorbed. The 2000 RDA states in humans "approximately 70%–90% of vitamin C is absorbed at moderate intakes of 30–180 mg/day. However, at doses above 1,000 mg/day, absorption falls to less than 50%" (12).
It goes on to explain that vitamin C Ascorbic acid is absorbed in the body by both active transport and passively by simple diffusion. So even if the vitamin C transporters become saturated, simple diffusion will still push vitamin C across the intestinal walls. Diffusion is the net movement of molecules or atoms from a region of high concentration to a region of low concentration because of random motion of the molecules.
According to the 2000 RDA for Vitamin C document, sodium-dependent active transport—sodium-ascorbate co-transporters (SVCTs) and hexose transporters (GLUTs)—are the two transporter proteins required for active absorption of vitamin C. SVCT1 and SVCT2 import the reduced form of ascorbate across plasma membranes. GLUT1 is a good intake valve for the dehydroascorbic acid (DHA) form of vitamin C. It is better at transporting DHA than Glucose.
I believe that a big part of the problem is that the NIH group may have ignored the passive absorption due to diffusion and confused "blood saturation" with "blood steady state." Please explain the difference.
Hickey: You are right, the NIH's initial problem was that they measured plasma levels 12 hours after giving the dose. They were measuring the steady state baseline level after the dose had been excreted. The minimum level rather than the maximum.
The National Academy is quoting a drop-off in absorption with high doses in healthy young people. This drop-off does not occur when a person is ill or stressed. In sick people the plasma vitamin C can quickly fall to near undetectable levels and massive intakes are needed to restore the body. The interesting question is what is going on when a person is stressed or sick and their intake increases by an order of magnitude or so.
Passwater: I have seen no other study to add to or clarify these data since your 2008 study (2). In fact, the only paper I have found to even discuss your data is the review by Dr. Jorge Duconge and colleagues (13). Have there been others?
Hickey: That's a difficult one. I can't speak for others but often their private comments are at odds with the publications. Only one scientist, from the Linus Pauling Institute of all places, has tried to defend the NIH work to me. In private many scientists find the NIH errors impossible to support but consider it might be professional suicide to challenge them. The attempt to destroy Linus Pauling's reputation when he supported high-dose vitamin C acts as a warning to others.
Passwater: OK, let's look at some of the specifics that the Dynamic Flow model tell us. Will a single dose of any amount of vitamin C have important biological effect?
Hickey: The pioneers using vitamin C for treating the sick explained that the doses needed to be large and frequent. To give examples, Drs. Fred Klenner, Robert Cathcart and Irwin Stone found that a patient needed doses repeated through the day. We now have a good understanding of why this is so.
The half-life of high dose vitamin C in the blood is very short perhaps half an hour. So, 30 minutes after a dose is absorbed half of it will be excreted. After one hour the blood level would be reduced to one quarter. This gave rise to the "expensive urine" myth but is actually how dynamic flow works.
A single large dose of vitamin C provides antioxidant electrons which it can supply free to the body. Free means the body does not need to use energy to provide the antioxidants to help it heal. Each molecule of vitamin C carries two antioxidant electrons, but they are used up quickly and need to be replaced by the next dose. If you think about it, once a vitamin C molecule has given up its electrons it is of no more use and it can be excreted in oxidized form or recycled (recharged) by synergistic antioxidants.
Passwater: What are the blood levels normally found in the general population by blood testing labs?
Hickey: It depends on the population and their state of health. Most people are measured at some fraction of the baseline level and are deficient. Some vegetarians and, of course, people who normally take high-dose supplements, have adequate levels provided they are in reasonably good health.
Passwater: Could measurement of blood levels of vitamin C be useful in establishing a meaningful RDA? What would the data suggest as a "healthy" blood level for vitamin C? Is there any tie-in with levels in ascorbate-producing animals?
Hickey: The whole idea of an RDA for vitamin C does not make sense. Firstly, we are dealing with a population who are largely deficient (below the baseline). Then there is biological variation, people have individual requirements. But the proverbial elephant in the room is bowel tolerance.
Suppose the NIH were correct and, say, 200 mg a day is sufficient for a healthy young man to maintain a baseline plasma level of 60-70 µM/L. He will occasionally feel tired, stressed or get a common cold and his plasma levels will drop dramatically, potentially to something close to zero. Now this stressed individual might need an intake of, say, 20,000-40,000 mg (20-40 grams) a day to maintain his levels. In other words, the RDA would place a sick person in a state of induced scurvy and to correct the problem requires increasing the intake by a factor of 100 or more. When stressed even a healthy young man might need to be consuming a year's worth of RDA intakes in a single weekend.
Passwater: What is the largest dose you have studied?
Hickey: Seventy-two grams of ascorbic acid and liposomal vitamin C over a period of a few hours in a healthy individual. However, I have known cancer patients who take similar amounts every day.
Passwater: Did any of the studies show kidney stones? I ask this because there's a myth that incorrectly suggests that vitamin C can cause kidney stones. The literature shows that vitamin C prevents kidney stones; it does not cause them (14).
Hickey: I have not come across kidney stones with oral intakes and the physicians and scientists who communicate with me have not reported the problem. There are some indications in the literature that kidney stones may be an issue with IVC, but the problem is overstated.
Passwater: Can we expect to see the rest of the scientific community starting to understand the dynamic flow model for vitamin C?
Hickey: The current pressure is for scientists working in the area to support the NIH claims that only low oral doses are absorbed and only IV can help cancer patients. One way of checking this is to ask how much vitamin C the researchers take themselves. Often, they publicly recommend relatively low intakes, say 500 mg or less, but take many times the amount themselves, several grams a day.
Passwater: OK, this begs the question of how much do you take?
Hickey: My minimum and typical daily intake is about 9 grams of ascorbic acid spread out over the day, but this can increase 5-10-fold if I feel a cold might be on the way.
Passwater: Dr. Hickey, thanks for sharing your research with us, and for your book "Ascorbate: The Science of Vitamin C." We will look forward to your new book on Vitamin C and Cancer.
Next month we will discuss whether or not we can further optimize the uptake of vitamin C into all cells with the "Multi-C Protocol" of Dr. Thomas E. Levy. WF

Dr. Richard Passwater is the author of more than 45 books and 500 articles on nutrition. Dr. Passwater has been WholeFoods Magazine's science editor and author of this column since 1984. More information is available on his website, www.drpasswater.com.
References
1. Cathcart, R. F. Vitamin C, titrating to bowel tolerance, anascorbemia, and acute induced scurvy. Med Hypotheses 7(11):1359-76 (1981)
2. Hickey, S., Roberts, H.J. & Miller, N.J. Pharmacokinetics of oral vitamin C. J. Nutr. Environ. 17(3):169-177 (Mar 2008) https://doi.org/10.1080/13590840802305423
3. Hickey, D. S., Roberts, H.J. and Cathcart R. F. Dynamic Flow: A New Model for Ascorbate. J. Med. 20(4):237-244 (2005). http://orthomolecular.org/library/jom/2005/pdf/2005-v20n04-p237.pdf
4. Passwater, R.A. I.V. Vitamin C and Cancer Research. Whole Foods (Mar 2018) https://wholefoodsmagazine.com/columns/vitamin-connection/v-vitamin-c-cancer-research/
5. M. Levine et al., "Vitamin C pharmacokinetics in healthy volunteers: evidence for a recommended dietary allowance," Proc. Natl. Acad. Sci, vol. 93, no. 1, p.3704-3709 (1996).
6. Hickey, S. and Roberts, H. "Ascorbate: The Science of Vitamin C," p. 82, Lulu.com Publ. ISBN: 97814116072486 (2007).
7. Padayatty, S.J, Sun, H., Wang, Y., et al., Vitamin C Pharmacokinetics: Implications for Oral and Intravenous Use. Ann Intern Med. 2004;140(7):533-537. DOI: 10.7326/0003-4819-140-7-200404060-00010]
8. Passwater, R.A. The Science of Vitamin C and Cancer. Whole Foods (December 2007) http://www.drpasswater.com/nutrition_library/
hickey.html
9. Passwater, R.A. The Inappropriateness of So-Called "Evidenced Based Medicine"—Especially for Studying Nutrients. A Tarnished Concept. Whole Foods (March 2012) https://wholefoodsmagazine.
com/columns/vitamin-connection/inappropriateness-so-called-evidenced-based-medicine-especially-studyin/
10. Murata A, Morishige F, Yamaguchi H. (1982) Prolongation of survival times of terminal cancer patients by administration of large doses of ascorbate, International Journal for Vitamin and Nutrition Research, Supplement, 23, 101-113.
11. Chen, Q., Espey, M. G., Krishna, M.C., et al. Pharmacologic ascorbic acid concentrations selectively kill cancer cells: Action as a pro-drug to deliver hydrogen peroxide to tissues. PNAS September 20, 2005. 102 (38) 13604-13609; https://doi.org/10.1073/pnas.0506390102.
12. National Academy of Sciences. Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids. Institute of Medicine. National Academy Press (2000)
13. Duconge, J., Miranda-Massari, J. R., Gonzalez, M. J., et al. Pharmacokinetics of Vitamin C: Insights into the oral and intravenous administration of ascorbate. PRHSJ 27(1):7-15 (2008)
14. http://orthomolecular.org/resources/omns/v09n05.shtml
Source: https://wholefoodsmagazine.com/columns/vitamin-connection/the-science-of-vitamin-c-research-on-optimizing-blood-and-cellular-levels/
